Dr. Anand Chandrasekhar

PhD, 1994 University of Iowa
Mechanisms regulating neuronal development and physiology in vertebrates
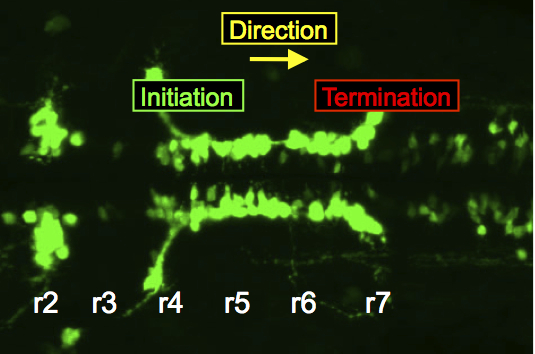
In the zebrafish hindbrain, FBM neurons migrate caudally from rhombomere 4 into rhombomeres 6 and 7.
During nervous system development in vertebrates, newborn neurons migrate extensively to precise locations where they participate in assembling circuits that control behaviors and functions essential for health and survival. Defective migration of neurons in different brain regions is associated with neuronal migration disorders that can lead to epilepsy, and cognitive and motor deficits. My lab is interested in understanding the cellular and molecular mechanisms regulating neuronal migration, and in examining the behavioral consequences of defective migration.
The migration of facial branchiomotor (FBM) neurons is an excellent model system to investigate neuronal migration mechanisms. My lab identified the first gene necessary for FBM neuron migration. We use the zebrafish and mouse models to dissect these mechanisms using genetic, biochemical and imaging approaches. While a large number of genes, including several functioning the Wnt/Planar Cell Polarity (PCP) signaling pathway, are required for FBM neuron migration, their mechanisms of action have remained largely obscure, in part because they may be independent of PCP signaling. Our recent work has provided insights into the roles of Wnt/PCP-associated transmembrane proteins Vangl2 and Celsr1 in respectively regulating the initiation and direction of migration of FBM neurons in the zebrafish and mouse hindbrain.
Current projects:
- Understanding the role of Vangl2 in initiating FBM neuron migration in zebrafish and mice: We propose that Vangl2 functions in the floor plate cells in rhombomere 4 to localize signaling molecules necessary for initiating migration. We are testing this model through loss-of-function experiments in zebrafish and mice, as well as by modulating the localization of Vangl2 in floor plate cells.
- Testing the role of candidate genes in FBM neuron migration in zebrafish: We have identified a promising set of candidate genes through expression profiling experiments. We are examining their roles in migration by generating loss-of-function alleles using the CRISPR/Cas9 method.
- Understanding the role of Celsr1 in directing FBM neurons to migrate caudally: We propose that in mouse Celsr1 blocks migration into anterior rhombomeres by suppressing the response of FBM neurons to rostrally expressed Wnts. We are testing this model through hindbrain explant studies and mutant analyses.
- Examining the behavioral and physiological consequences of defective migration: We propose that defective migration of FBM neurons results in improper assembly of the branchiomotor circuits regulating feeding. We have developed methods to measure food intake in zebrafish and are examining feeding efficiency in neuronal migration mutants. In collaboration with the Fetcho lab (Cornell), we will examine the effects on neuronal physiology and connectivity in migration mutants.
Other Projects
- Analysis of the role of integrin signaling in FBM neuron migration.
- Forward genetic screen for genes regulating neuronal development in the zebrafish hindbrain (collaboration with S. Ekker and K. Clark, Mayo Clinic).
- Establish of an in vitro (cell culture) system using rhombomere explants to study the roles of Wnt/PCP components in migration.
Richard F. and Sharon A. Keister Faculty Enhancement Award in Biological Sciences 2019
William T. Kemper Fellowships for Teaching Excellence 2008
Purple Chalk Award 2005, 2007
A list of Dr. Chandrasekhar's publications is available from Google Scholar.