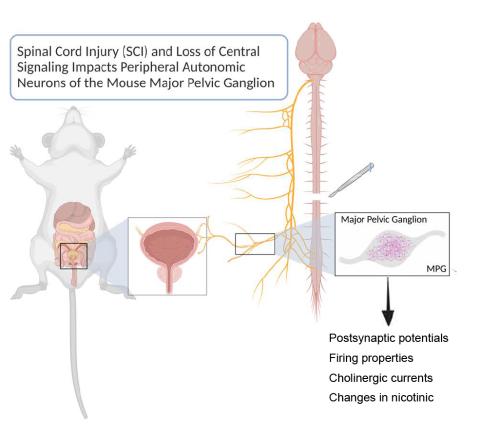
Study sheds light on overlooked effects of spinal cord injuries on downstream neurons
More than 80% of Americans living with a spinal cord injury experience some form of bladder dysfunction. Because of its negative impact on their long-term health and social well-being, restoration of bladder function is often prioritized over being able to walk again by persons living with spinal cord injuries. Yet, the focus of most spinal cord injury research is on paralysis and motor dysfunction.
In a new study published online this month in the Journal of Neurophysiology, a team of Mizzou biologists report on the off-target effects of spinal cord injury on the uninjured neurons that control the bladder. Their study shows changes in the properties and expression of a subset of neurotransmitter receptors that lead to fundamental changes in the synaptic properties of these neurons. The findings have implications for the design of new therapeutic approaches that aim to restore bladder function after such injuries.
The team included Dr. Cindy Kyi (PhD, ‘18), who carried out the study while a graduate student in the Division and is lead author, Dr. David Schulz, Virginia Garcia, and Dr. Michael Garcia.
The following is a brief interview with Dr. David Schulz about the study and its findings.
What drew you to this line of research?
I started out like many other neuroscientists wanting to make people walk again, and then realized there was a huge disconnect between what people wanted and what was being studied. If you go into PubMed and search for “bladder spinal cord injury,” you’ll come up with papers at a rate that’s probably 20, 50, or even 100 times less than paralysis. It is deeply understudied. Yet in the clinical literature, individuals with spinal cord injuries far prioritize recovery of some bladder function, bowel function, and sexual function over being able to walk again. So there’s not only a discrepancy in the amount of research, but it is exactly inverse of what the actual patient population wants us to be studying.
What do you think accounts for this discrepancy in research?
In general, I think that for too long we have assumed that the uninjured parts of the nervous system go into a state of suspension after an injury and just wait to get reconnected. But there is so much constant feedback in these neural systems that when you have a severe disruption, like in a spinal cord injury, the loss of that feedback triggers a push back to try to restore function. That triggering never resolves because the injury itself is permanent, so therefore what is normally compensatory and potentially even restorative spirals out of control and becomes pathological. The worry is that if we don’t monitor what’s changing downstream of injuries and think about whether those need to be part of the therapeutic regimen (in addition to fixing the injury), then we might reconnect the brain to a fundamentally altered lower nervous system and still not restore function. And let’s face it: bladder and bowel isn’t really that “sexy” relative to seeing a paralyzed patient walk again. But it’s so important, for the welfare of the patient and for their long-term health consequences.
What is the big question this paper asks?
What we did in this study was ask do neurons that are not even in the central nervous system fundamentally change even though they’re not injured? The neurons we looked at are out in the periphery, near the bladder itself. The follow up question is how do they change? And, if we were to reconnect, will they change back on their own or do we need to do something to either encourage them to change or fool them into thinking everything is OK and not change while we while we restore the injured part of the nervous system?
We use bladder as a model to ask this question, but this question applies to any system where there is change in the fundamental properties of neurons in the nervous system below an injury or a disease focal point. So the work extends beyond bladder and beyond spinal cord injury.
What were the “nuts and bolts” of your study?
We used a combination of molecular and electrophysiological experiments to characterize the synaptic properties of the neurons that innervate the bladder in mice, namely the major pelvic ganglion. We first characterized the physiology and receptor identities of these neurons in a normal/uninjured state. We then determined if and how these properties were altered after an acute (3 days post injury) and chronic (28 days poster injury) spinal cord injury.
What did you find and why is it significant?
We found that, yes, these neurons do change. Peripheral neurons receive signals from the central nervous system through chemicals called neurotransmitters. These neurotransmitters are received by receptors. What seems to be changing is that the peripheral neurons are changing the properties of and the expression of those transmitter receptors and it’s resulting in synaptic changes between the central nervous system and these peripheral neurons. The synaptic property changes are interesting and exciting because that’s very easy to monitor from a gene expression/receptor expression point of view. When we do get into intervention and therapy, we have an endpoint that can be monitored and know if the intervention prevents or reverses these changes. A secondary finding is that there is a subset of receptors that change, and these might give us more specificity with drugs to target bladder dysfunction. If we can figure out that there’s a bladder specific or an autonomic specific subset that changes, then our pharmacology could get much more specific and hopefully lead to a treatment that enhances bladder function without widespread side effects.
We also saw very different changes in the receptors immediately following the injury and at the chronic timepoint. The time frame is important and meaningful for a few reasons. If you want to help people who have been injured for a while, you’re dealing with a chronic state, and if you want to think about immediately deploying therapeutics during trauma that’s the acute state. If all we know are the acute effects, we can’t do anything to move the needle for those people who have been living with spinal cord injury and bladder dysfunction for many years. We also need to know how those differences are related so that if we were to employ a therapy immediately, we need to know whether that changes the endpoint of chronic injury or not. We can’t assume those are totally independent or are similar.
What do the findings portend for treatment of bladder problems and other secondary complications of spinal cord injuries?
The cutting edge of therapy is in neurostimulation. There are already FDA-approved therapeutics to implant electrodes near or at the spinal cord for bladder dysfunction/relief. I am excited about being able to really fine-tune neurostimulation in ways that understand the progression of the disease and where we might intervene. My dream (and others) is closed loop neuroprosthetics. Closed loop means that there is both monitoring of the bladder and stimulation of the nerves by the device. Knowing what changes and knowing what normal activity looks like in these neurons allows us to participate in informing and designing those closed loop neuroprosthetics. Ideally, we would be able to artificially bypass the spinal cord injury and restore sensation of bladder fullness, which would have a profound effect on people with spinal cord injuries by reducing the risk of autonomic dysreflexia and unchecked UTIs, both of which are dangerous consequence of bladder dysfunction in persons with spinal cord injury. We’re really trying to push towards having long term impacts of this work.
Read the full study: Kyi, C.W., Garcia, V.B., Garcia, M.L., Schulz, D.J. Spinal cord injury is associated with changes in synaptic properties of the mouse major pelvic ganglion. Journal of Neurophysiology, 07 SEP 2022. https://doi.org/10.1152/jn.00477.2021.